Richard GAO
Trademark attorney, attorney-at-law
Trademark attorney, attorney-at-law
As a special product, the quality of drugs is a matter of health and safety of millions of consumers. Therefore, China has implemented strict regulations on drugs, including their names. There are three different names for drugs: the generic name, trade name, and trademark, each with its own concepts, functions, and usage.
I. The generic name of drugs
The generic name of drugs is the statutory name of drugs formulated by the China Pharmacopoeia Committee in accordance with specific rules and featured by generality. Drugs must prominently display the generic name. The generic name of the drug is the most prominent element in the drug packaging, for example, on the packaging of the common cold medication “白加黑”, the generic name of the drug is prominently displayed as “Aminophenol pseudo-malmefene tablets Ⅱ / Aminphenomel tablets”.

Given that the generic name of a drug is a statutory name with public nature, it cannot be registered and used exclusively as a trademark by a specific entity. According to the provisions of the Drug Administration Law, if a name has been used as a generic name of a drug, it shall not be used as a drug trademark. The English and Chinese translations of the generic name and the word stem of special term in the International Nonpropietary Names for Pharmaceutical Substances (INN) shall also not be used as drug trade names or drug trademarks.
Since the generic name of a drug lacks the distinctiveness required for trademark registration, the trademark application using a generic name of a drug inevitably violates the Paragraph (1)(a) of Article 11 of the Trademark Law. In the trademark examination, if the applied trademark is found by the examiner to be identical or similar to the drug’s generic name, the generic name and the word stem of special term in International Nonpropietary Names for Pharmaceutical Substances (INN), it will be rejected, not registered or declared invalid according to law.
The following cases are examples:
| Trademark | Related decisions | |
|
|
Venetoclax | "Venetoclax" is the generic name of the drug for the treatment of leukemia. |
|
|
依维莫司 | This trademark is used on the product of “drugs for the treatment of cancer” and simply represents the generic name of the product. |
|
|
Omadacycline | “omadacycline” is included in INN recommended by the WHO Drug Information and has become the generic name of antibiotic drug. |
|
|
LAROTINIB |
‘TINIB’ can be translated as '替尼', which refers to enzyme inhibitors. As the word stem of the generic name in INN, it is used in the treatment of oncology. |
II. Trade name of drug
Although the generic name of a drug is the most important part of drug labeling, it is often obscure and difficult for the public to remember. Therefore, in the real life, the drug’s trade name that is easy to read and remember came into being.
Drug’s trade name is the name of a registered legal name created by an enterprise that distinguishes it from other enterprises of the same product and is characterized by its exclusiveness nature. The drug’s trade name reflects the source of the drug manufacturer and its exclusive right to the trade name. Therefore, it should not be identical or similar to the drug trade names used by others, and must also be approved by the relevant authorities before use.
It can be seen that both drug trade names and drug trademarks have the function of distinguishing drug producers. The drug trade names can be registered as trademarks under the condition that they comply with the relevant provisions of the Trademark Law, while the drug trademarks can only be approved by the relevant authorities for the use as drug trade names under the condition that they comply with the provisions of the Regulations on Drug Trade Name Naming and other drug regulatory laws.
Unlike trademarks, drug’s trade name can only be composed of Chinese characters, and shall not use graphics, letters, numbers, symbols and other marks. Moreover, there are also some special provisions on the use of drug trade names. For instance: drug trade names shall not be written on the same line with the generic name, its font and color shall not be more prominent than the generic name, and the font size for a single word shall not be greater than one-half of the font used in the generic name, etc.

On the packaging of another common cold medication, “新康泰克”, for instance, the trade name “新康泰克” and the generic name “Aminomethylamine Tablets (II)” are separated by two paragraphs, and the trade name is substantially smaller than the generic name.
III. Drug trademark
Compared with drug trade names, drug trademarks contain a greater variety of constituent parts: words, graphics, letters, numbers and other elements and their combinations. Therefore, trademarks on drug labels can enrich label’s content, making it easier for consumers to identify the source of the product.
In terms of use, The Regulations on the Administration of Drug Instructions and Labeling provides: If a drug label contains a registered trademark, it shall be printed in the corners of the drug label; if text is contained, the font size for a single word shall not exceed one-fourth the front size used for generic names. Therefore, the drug trademarks that are not a trade name are placed in the corners of drug labels and are not significant.
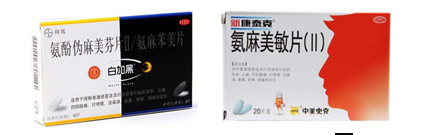
For example, the mark Bayer, , SK&F, , and on the aforementioned two drug packages are the registered trademarks. And these drug trademarks are in the corners of the package, and the font is smaller than that of the generic name and trade name, rendering them less recognizable.
In view of this, many enterprises register the drug’s trade names as trademarks, which make it easier for consumers to identify the brand in actual use and also help to strengthen the protection of the brand. For example, the drug trade names “新康泰克” and “白加黑” are also registered trademarks of the relevant enterprises.
So is it required to register a drug trademark? The author says yes. Article 6 of the Trademark Law provides that “The goods for which the laws and administrative regulations require a registered trademark shall apply for trademark registration; without the approved registration, it shall not be sold in the market.” However, the Drug Administration Law implemented in 2019 does not explicitly make mandatory provisions for the registration of drug trademarks. The Judgment Standards on General Illegal Act on Trademark implemented by the CNIPA last year only requires trademark registration for the production and sale of tobacco products such as cigarettes, cigarillos, packaged tobacco and electronic cigarettes but no drugs. However, in the drug industry, drug trademark registration is still required. At present, some regulations governing drug administration still retain the requirements of the drug trademark registration. The Regulations on the Administration of Drug Instructions and Labeling, for example, provides that “Unregistered trademarks and other drug names not approved by the State Food and Drug Administration are prohibited in drug instructions and labels.” Therefore, in the actual drug administration and regulation, drug trademark registration is still required in practice. Moreover, drug trademark can also enhance the protection of drug brands and make it easier for consumers to identify the source of production.
In summary, the use of generic name, trade name and trademarks of drugs should comply with the relevant legal provisions in the drug and trademark field at the same time. Pharmaceutical companies should register drug trade names and drug trademarks as early as possible to build and expand their brand portfolio, so as to gain the upper hand in the marketplace and obtain the necessary legal protection.
